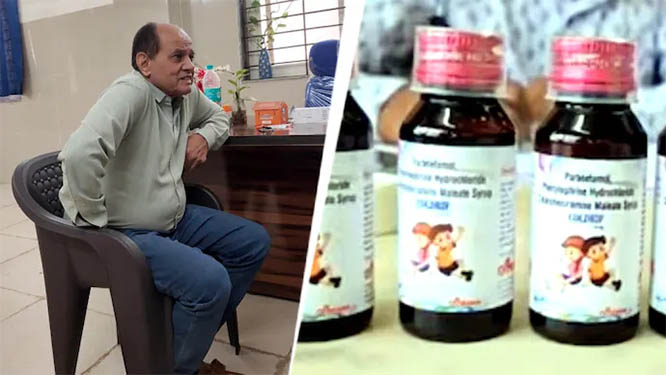
Bhopal / Chennai / New Delhi, Oct 5: A massive outcry has erupted after 14 children died in Madhya Pradesh’s Chhindwara district, allegedly after consuming a toxic batch of Coldrif cough syrup. Authorities have arrested Dr. Praveen Soni, a government-appointed physician practising in Parasia, who allegedly prescribed the syrup to the children.
According to official reports, most of the children had initially shown mild cold and fever symptoms in early September. However, after taking the syrup, their conditions deteriorated rapidly — with symptoms of kidney failure and reduced urine output. Laboratory tests later confirmed the presence of diethylene glycol, a highly poisonous chemical, in the syrup.
The Madhya Pradesh government has now banned Coldrif and another syrup, Nextro-DS, across the state, and announced ₹4 lakh compensation for the families of the deceased. Chief Minister Mohan Yadav called the deaths “extremely tragic” and vowed strict action against those responsible.
“The sale of this syrup has been banned across Madhya Pradesh. A ban is also being imposed on other products of the company that manufactures it,” the CM said on X (formerly Twitter).
The syrup in question — Coldrif (Batch No. SR-13) — was manufactured by Sresan Pharmaceuticals, Kancheepuram, Tamil Nadu. A test conducted at the Drug Testing Laboratory in Chennai found that it contained 48.6% diethylene glycol (w/v) — a toxic industrial solvent known to cause kidney failure.
Following the revelation, Tamil Nadu authorities declared the batch “Not of Standard Quality” and banned its sale. The Central Drugs Standard Control Organisation (CDSCO) has launched risk-based inspections at 19 pharmaceutical units across six states, including those producing cough syrups and antibiotics.
Samples from the victims and the syrups have been sent to the National Institute of Virology (Pune) and AIIMS-Nagpur for further analysis. A multi-agency team — including experts from the ICMR, NEERI, and CDSCO — is investigating the cause of deaths.
The tragedy has revived global concerns about India’s drug safety. In 2022, the World Health Organization linked cough syrups made by another Indian firm to the deaths of 70 children in Gambia, though India later contested the findings.
As the investigation deepens, authorities have seized remaining Coldrif stocks and placed Sresan Pharmaceuticals under scrutiny for potential violations of the Drugs and Cosmetics Act, 1940.






Comments
Add new comment